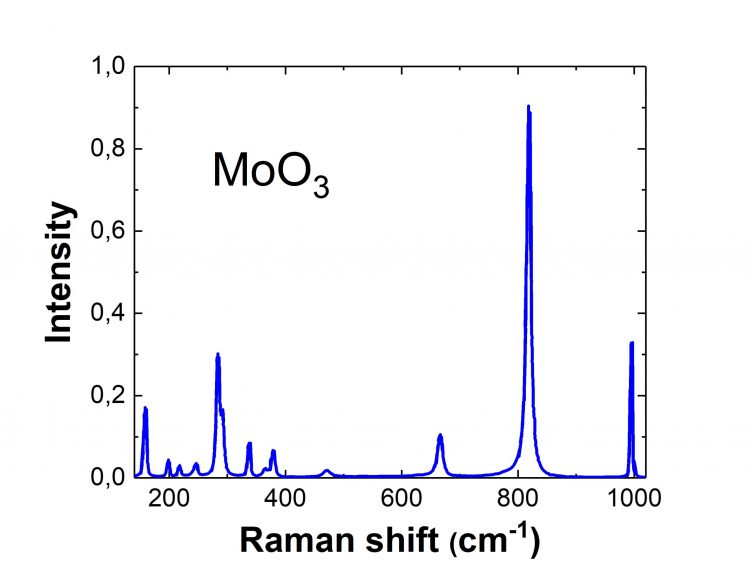
Molybdenum Trioxide Raman Spectrum
In general, molybdenum oxides are classified into two types: the metastable monoclinic β-MoO3 phase and the thermodynamically stable orthorhombic α-MoO3 phase. The latter kind, in particular, is an extremely promising oxide with structural anisotropy. The orthorhombic α-MoO3 phase is a wide bandgap n-type semiconductor, which makes it a very attractive material for different technological applications. For example, it can be used as photochromic materials that change from colorless to blue by UV irradiation, smart windows, where Au nanoparticles improve the visible-light coloration performance of the MoO3 thin film, self-developing photography, conductive gas sensors, lubricants, and catalysts.
Contact us to get access to Raman Spectra Database more than 20 000 chemical and biological substances
Raman spectroscopy of MoO3
Orthorhombic α-MoO3 is composed of MoO6 octahedral corner-sharing chains, with the edge shared by two similar chains to form the layers bonded by the weak van der Waals attraction. Thus, MoO3 Raman spectra have 12 signature peaks related to stretching, bending, wagging, and twisting modes. The record of these peaks allows the evaluation of the sample quality. Hence, such an accurate and simple quality-control method makes the use of MoO3 promising for various applications.
Raman Spectra Library
Raman spectroscopy can uniquely identify many chemical and biological agents.
All substances