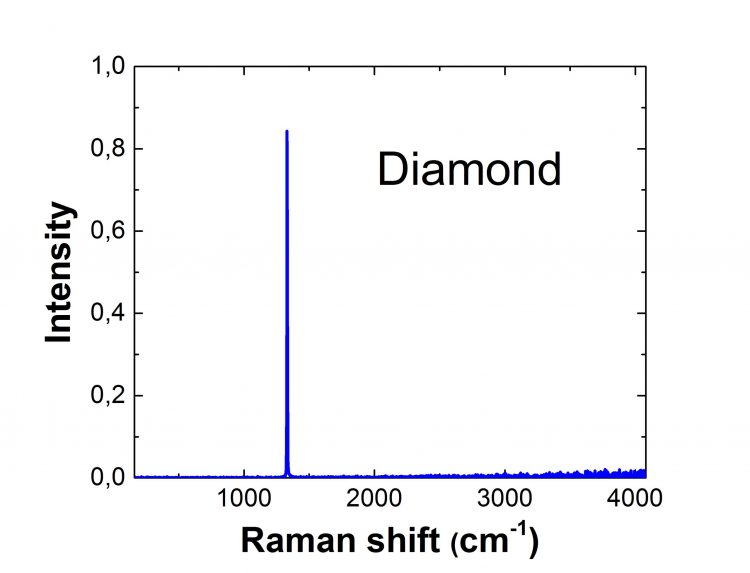
Diamond Raman Spectrum
Diamond is a type of carbon with atoms arranged in a diamond cubic crystal structure, with eight atoms per unit cell. This lattice comprises two interpenetrating face-centered cubic lattices, one of which is displaced by 1/4 of a cubic cell's diagonal. Diamond has the highest hardness and thermal conductivity of any natural substance, qualities that make it ideal for cutting and polishing equipment in the industry. Diamond also has a high refractive index, which makes it exceedingly shiny, and a high optical dispersion, making it possible to disperse light of different colors. For this reason, jewelry with diamonds is luxurious and costly.
Contact us to get access to Raman Spectra Database more than 20 000 chemical and biological substances
Raman spectroscopy of Diamond
Diamonds are also extremely expensive due to their rareness. Diamond Raman spectra exhibit eight distinct monochromatic frequencies. Only one of these is active, and it corresponds to the triply degenerate vibration of the carbon atoms' two Bravais lattices regarding each other. The remaining seven are inactive and extremely small compared with the active mode of vibration. Thus, in most cases, diamond Raman spectra reveal one intensive line. Raman spectroscopy is an excellent tool for quickly identifying whether a diamond is natural or not because there are more photoluminescence centers in synthetic diamonds than in natural diamonds.
Raman Spectra Library
Raman spectroscopy can uniquely identify many chemical and biological agents.
All substances