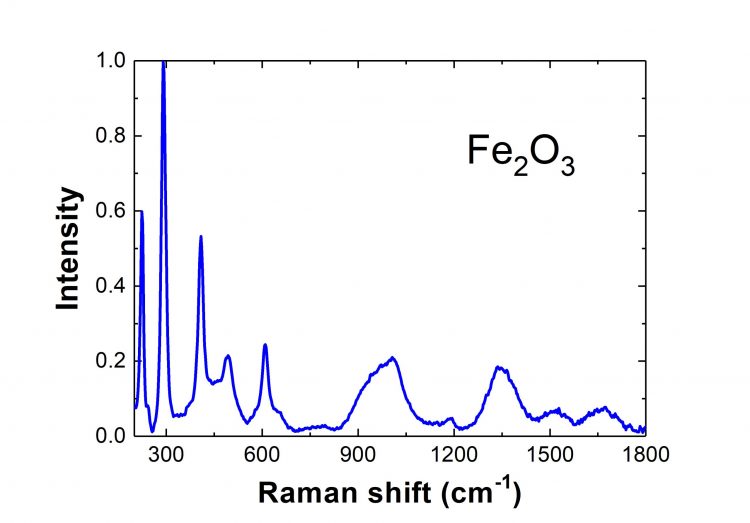
Hematite Raman Spectrum
Fe2O3 is widely known as hematite – the primary source of iron for the steel production industry. Its most common form, α-Fe2O3, has a rhombohedral structure. Below 260 K, this compound is antiferromagnetic, whereas above this temperature, it exhibits weak ferromagnetism, up to 950 K (Néel temperature). The synthesis of hematite particles (α-Fe2O3) of different shapes and sizes has been studied thoroughly because of their new physical and chemical properties compared to bulk materials. Fe2O3 nanoparticles are also of great interest due to their potential applications in inorganic pigments, catalysts, humidity and gas sensors, photoelectrolysis reactors, photoanodes in photo-electrochemical cells, water treatment, lithium-ion batteries, etc.
Contact us to get access to Raman Spectra Database more than 20 000 chemical and biological substances
Raman spectroscopy of Fe2O3
Pure Fe2O3 belongs to the R-3c crystal space group. Hence, according to group theory, the pure hematite Raman spectrum is expected to contain seven phonon lines. Also, the shifts in the spectral peaks reveal the difference in the shapes and sizes of the nanoparticles. Therefore, Raman spectroscopy is a useful tool for characterizing Fe2O3 nanoparticles.
Raman Spectra Library
Raman spectroscopy can uniquely identify many chemical and biological agents.
All substances